Electrochemistry is the part of science which deals with the interrelationship of electrical currents, or voltages, and chemical reactions, and with the mutual conversion of chemical and electrical energy.
Electrolyzed Water (EW) Technology is based on a new, previously unknown law of anomalous changes of reaction and catalytic abilities of aqueous solutions subjected to electrochemical unipolar (either anodic or cathode) treatment. EW is necessarily associated with alteration of its chemical composition, acidity and (or) alkalinity within a wide range.
That is why EW application makes it possible:
- to exclude from routine technological processes the regulation of solution properties with costly reagents;
- to improve quality of the treated substances;
- to reduce the number and duration of technological operations;
- to decrease their labour-consuming nature;
- to facilitate and simplify processes of water and sewage purification.
Unlike well-known electrochemical reactions, in the processes of electrochemical activation initial substances are diluted aqua-saline (brine) solution and mains water. The eventual EW products are not concentrated chemical substances, but activated (Anolyte and Catholyte) solutions: low mineralised liquids in a metastable state, manifesting increased chemical activity. Synthesis of electrochemically activated solutions is only possible when unipolar electrochemical exposure is combined with treatment of as many as possible micro volumes of liquid in high voltage electric field of a double electric layer near the electrode’s surface.
The above stated conditions of producing activated solutions can be realized only in special diaphragmatic cells (round or square) which are the key elements of every Envirolyte or ECO unit.
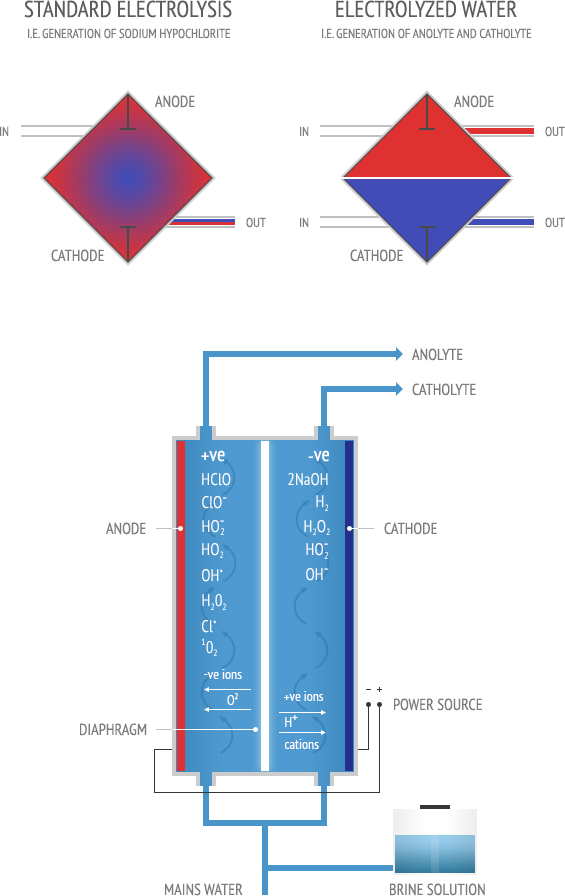
Drawing of Envirolyte operational principle
The EW solutions produced by Envirolyte units (Anolyte and Catholyte) are channelled through canals and chambers and separated by the membranes. This unique patented process allows for more even distribution of electrolyte (brine solution) within the volumes of the chambers and reduces the risk of the formation of stagnant zones when flow rates of electrolyte are high. The construction of the diaphragmatic cells also allows highly effective evacuation of products of electrochemical and chemical reactions from the chambers.

