BY PRODUCTS TESTING
- Independent testing
Envirolyte systems and generated solutions (anolyte and catolyte) have been put through extensive series of independent tests and on-site trials including hospitals, breweries, water systems and agricultural environments. - Safer world
Being environmentally friendly, completely safe, non-toxic and non-irritant Anolyte is welcome where traditional chemicals fail to produce the desired results or can’t be applied at all. - Better alternative
The conclusions prove that Anolyte being a low-cost and powerful disinfectant is set to become the preferred solution for many sterilisation, disinfecting and water purification procedures.
Undertaken by Insitute of Chemistry of Tallinn Technical University, Estonia.
The aim of this study was to check the presence of toxic disinfecting by-products, chlorite (ClO2) and chlorate (ClO3) in the anolyte.
Anolyte was produced according to the instructions given by representatives of Envirolyte Industries International Ltd. Two different regimes were tested and according to these regimes anolyte is called Anolyte Medium (pH=2-3;ORP і 1100mV; Cac± 300mg/l) and Anolyte Strong (pH=2-3;ORP і 1100mV; Cac± 500mg/l). The anolyte for examination was produced immediately before test.
Ion chromatography was used for determination of chlorates and chlorites. The experiments were carried out utilising Ion Chromatograph CVET-3007 with the analytical column 6×600 mm filled with sorbent HIKS-1. 2.4 mM Na2-CO3 aqueous solution was used for dilution. Flow rate was 4.5 ml/min and the pressure 40-50 atm. The solutions of NaClO3 with the concentration of ClO3 -ion of 29.15 and 7.29 mg/l were used as standards.
The preliminary runs demonstrated that under conditions mentioned above ClO3 – ion could be separated successfully from chloride (Cl-) and determined at the level down to 0.5 mg/l. Chlorates were determined directly in the diluted anolyte, the chlorites were detected in diluted anolyte after the heating at 100o C for 5 minutes to convert chlorites to chlorates.
Anolyte Medium was diluted 1:200, 1:100 and 1:20. No peak responsible for the presence of any chlorate was observed in dilutions. The tests with heated samples diluted the same way showed the presence of chlorate neither (see chromatograms 1-3) Anolyte Strong was diluted 1:200, 1:100 and 1:20. No peak responsible for the presence of chlorate was observed in any dilutions.
The tests with heated samples diluted the same way showed the presence of chlorate nether (see chromatograms 4-6).
|
|
|
|
||
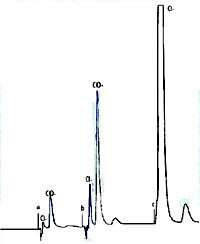
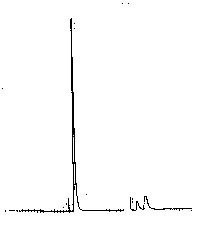
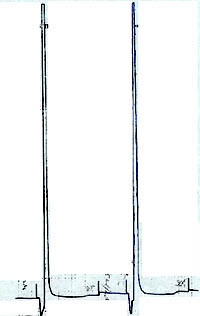
| Chromatogram 1. The ion chromatograms: a-standard (ClO3) with concetration 7.29 mg/l; b – standard (ClO3) with concentration 29.5 mg/l; c- sample anolyte medium 1:20 | Chromatogram 2. The ion chromatograms: a-sample (anolyte medium 1:200); b – standard (ClO3) with concentration 29.5 mg/l | Chromatogram 3. The ion chromatograms: a-sample (anolyte medium 1:100); b – anolyte medium 1:200. SO42- ion was added to the sample to ascertain the efficiency of chromatograohic conditions. | ||
|
|
|
|
||
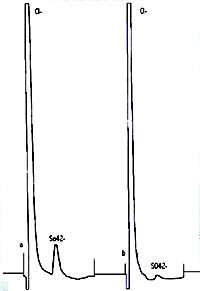
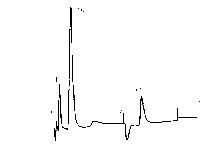
| Chromatogram 4. The ion chromatograms: a-standard (ClO3) with concetration 29.5 mg/l; b – standard (ClO3) with concentration 7.29 mg/l | Chromatogram 5. The ion chromatograms: a,b – sample anolyte strong 1:200 | Chromatogram 6. The ion chromatograms: a-sample (anolyte strong 1:20); b – anolyte strong 1:20. SO42- ion was added to the sample to ascertain the efficiency of chromatograohic conditions. |